Oxygen is the second largest component of the atmosphere, comprising 20.8% by volume. Liquid oxygen is pale blue and extremely cold. Although nonflammable, oxygen is a strong oxidizer. Oxygen is necessary to support life.
Oxygen will react with nearly all organic materials and metals, usually forming an oxide. Materials that burn in air will burn more vigorously in oxygen. Equipment used in oxygen service must meet stringent cleaning requirements, and systems must be constructed of materials that have high ignition temperatures and that are nonreactive with oxygen under the service conditions. Vessels should be manufactured to American Society of Mechanical Engineers (ASME) codes and designed to withstand the process temperatures and pressures.
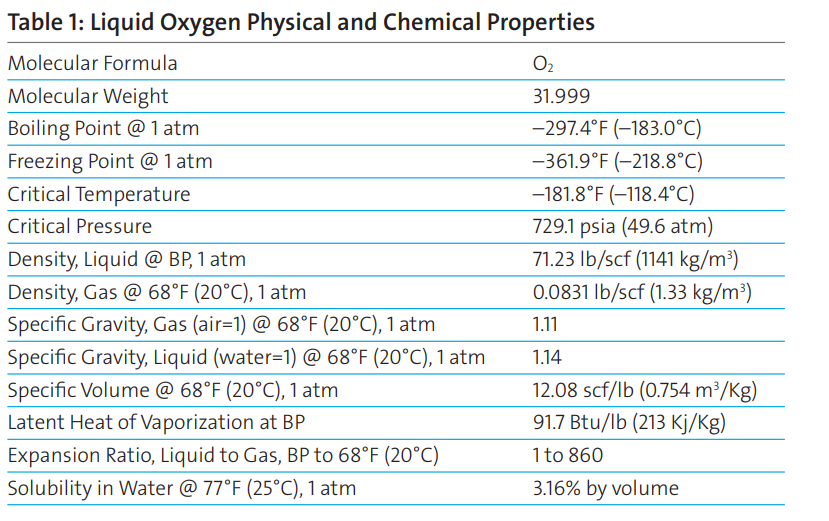
Liquid oxygen is a cryogenic liquid. Cryogenic liquids are liquefied gases that have a normal boiling point below –130°F (–90°C). Liquid oxygen has a boiling point of –297°F (–183°C).
Because the temperature difference between the product and the surrounding environment is substantial—even in the winter—keeping liquid oxygen insulated from the surrounding heat is essential. The product also requires special equipment for handling and storage.
Oxygen is often stored as a liquid, although it is used primarily as a gas. Liquid storage is less bulky and less costly than the equivalent capacity of high-pressure gaseous storage. A typical storage system consists of a cryogenic storage tank, one or more vaporizers and a pressure control system. The cryogenic tank is constructed, in principle, like a vacuum bottle. There is an inner vessel surrounded by an outer vessel. Between the vessels is an annular space that contains an insulating medium from which all the air has been removed. This space keeps heat away from the liquid oxygen held in the inner vessel. Vaporizers convert the liquid oxygen into a gaseous state. A pressure control manifold then controls the gas pressure that is fed to the process or application.
Vessels used in liquid oxygen service should be designed for the pressure and temperatures involved. Piping design should follow similar design and conform to national standards and codes.
Manufacture
Oxygen is produced by an air separation unit (ASU) through liquefaction of atmospheric air and separation of the oxygen by continuous cryogenic distillation. The oxygen is then removed and stored as a cryogenic liquid. Oxygen can also be produced noncryogenically using selective adsorption processes to produce gaseous product.
The ASU manufacturing process begins with a main air compressor and ends at the output of the product storage tanks. Air is compressed and sent through a cleanup system where moisture, carbon dioxide, and hydrocarbons are removed. The air then passes through heat exchangers where it is cooled to cryogenic temperature. Next, the air enters a high pressure distillation column where it is physically separated into a vaporous form of nitrogen at the top of the column and a liquid form of “crude” oxygen (~90% O2) at the bottom.
This crude oxygen liquid is withdrawn from the column and sent to a low-pressure column, where it is distilled until it meets commercial specifications. The liquid oxygen is sent to a cryogenic storage tank.
Uses
Oxygen is generally liquefied so that it can be more effectively transported and stored in large volumes. However, most applications use oxygen after it is vaporized to the gaseous form. The primary uses of oxygen relate to its strong oxidizing and life-sustaining properties. Oxygen is commonly relied upon in health and medical applications. Liquid oxygen is used as an oxidant for liquid fuels in the propellant systems of missiles and rockets.
Oxygen is widely applied in the metal industries in conjunction with acetylene and other fuel gases for metal cutting, welding, scarfing, hardening, cleaning and melting. Steel and iron manufacturers also extensively use oxygen or oxygen-enriched air to affect chemical refining and heating associated with carbon removal and other oxidation reactions. Benefits such as fuel and energy savings plus lower total emission volumes are often achieved when air is enriched or replaced with higher-purity oxygen.
In the chemical and petroleum industries, oxygen is used as a feed component to react with hydrocarbon building blocks to produce chemicals such as alcohols and aldehydes. In many processes, the oxygen for reaction can be obtained from the use of air. However, direct use of oxygen, or enrichment of the air with oxygen, is necessary for some processes. There are several major petrochemical intermediates that are presently manufactured with high-purity oxygen, including ethylene and propylene oxide (antifreeze), vinyl chloride (for PVC), and caprolactam (for nylon).
The pulp and paper industry uses oxygen as a bleaching and oxidizing agent. A variety of process (liquor) streams show enhanced physical properties after treatment with oxygen; plant operating costs also improve.
Similarly, oxygen enhances the combustion process in industries that manufacture glass, aluminum, copper, gold, lead, and cement, or that are involved in waste incineration or remediation. There are corresponding productivity, energy, maintenance, and emissions benefits end users may realize.
Wastewater treatment plants successfully employ oxygen to enhance their chemical process efficiency. Aquaculturists such as fish farmers also see benefits in the health or size of their livestock when the host environment is oxygenated.
Health Effects
Normally, air contains 21% oxygen, and oxygen is essentially nontoxic. No health effects have been observed in people exposed to concentrations up to 50% at 1 atmosphere for 24 hours or longer.
The inhalation at 1 atmosphere of 80% oxygen for more than 12 hours can cause irritation of the respiratory tract, progressive decrease in vital capacity, coughing, nasal stuffiness, sore throat, and chest pain, followed by tracheobronchitis and later by pulmonary congestion and/or edema. Inhalation of pure oxygen at atmospheric pressure or less can cause pulmonary irritation and edema after 24 hours.
Respiratory symptoms can occur in two to six hours at pressures above 1 atmosphere. One of the earliest responses of the lung is accumulation of water in its interstitial spaces and within the pulmonary cells. This can cause reduced lung function, which is the earliest measurable sign of toxicity. Other symptoms include fever and sinus and eye irritation.
When pure oxygen is inhaled at pressures greater than 2 or 3 atmospheres, a characteristic neurological syndrome can be observed. Signs and symptoms include nausea, dizziness, vomiting, tiredness, light-headedness, mood changes, euphoria, confusion, incoordination, muscular twitching, burning/tingling sensations (particularly of the fingers and toes), and loss of consciousness. Characteristic epileptic-like convulsions, which may be preceded by visual disturbances such as loss of peripheral vision, also occur. Continued exposure can cause severe convulsions that can lead to death. The effects are reversible after reduction of oxygen pressure.
Premature infants placed in incubators to breathe oxygen in concentrations greater than in air can develop irreversible eye damage. Within six hours after an infant is placed in a high-oxygen atmosphere, vasoconstriction of the immature vessels of the retina occurs, which is reversible if the child is immediately returned to air, but irreversible if oxygen-rich therapy is continued. Fully developed blood vessels are not sensitive to oxygen toxicity.
Extensive tissue damage or cryogenic burns can result from exposure to liquid oxygen or cold oxygen vapors.
Containers
Liquid oxygen is stored, shipped, and handled in several types of containers, depending upon the quantity required by the user. The types of containers in use include the dewar, cryogenic liquid cylinder, and cryogenic storage tank. Storage quantities vary from a few liters to many thousands of gallons. Since heat leak is always present, vaporization takes place continuously. Rates of vaporization vary, depending on the design of the container and the volume of stored product. Containers are designed and manufactured according to the applicable codes and specifications for the temperatures and pressures involved.




